Medical cold chain transport as a pharmaceutical cold chain logistics and temperature-controlled healthcare delivery system, explaining how medical cold chain transport works, is a specialized logistics system designed to store and move temperature-sensitive medical products, such as vaccines, biologics, insulin, blood plasma, and diagnostic reagents, within strictly controlled temperature ranges from origin to final destination. The goal is simple but critical: maintain product safety, potency, and regulatory compliance at every stage of transit.
Unlike standard logistics, medical cold chain transport integrates validated packaging, refrigerated vehicles, real-time temperature monitoring, and documented handling procedures to prevent temperature excursions that could render medical products ineffective or unsafe.
Understanding the Medical Cold Chain: The Big Picture
At its core, the medical cold chain is a continuous, unbroken temperature-controlled flow. If the temperature deviates, even briefly, the integrity of the product can be compromised.
Typical temperature ranges include:
| Category | Temperature Range | Examples |
|---|---|---|
| Frozen | -25°C to -15°C | Certain vaccines, plasma |
| Refrigerated | 2°C to 8°C | Vaccines, insulin, biologics |
| Controlled Room Temp | 15°C to 25°C | Some pharmaceuticals |
According to WHO and industry data, over 25% of vaccines globally are degraded due to cold chain failures, highlighting the importance of robust transport systems.
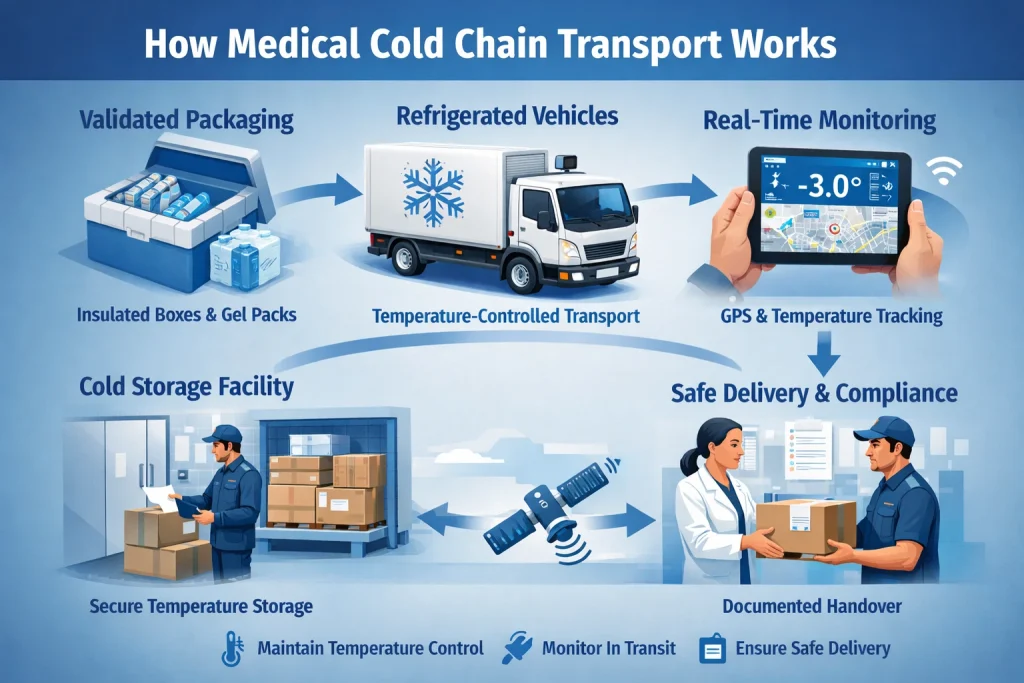
How Medical Cold Chain Transport Works (Step by Step)
1. Temperature Risk Assessment & Planning
Before transport begins, logistics providers analyze:
- Product temperature sensitivity
- Transit duration and route risks
- External climate conditions
- Regulatory requirements (GDP, ISO, MOH guidelines)
This planning stage determines whether active (refrigerated vehicles) or passive (insulated packaging) solutions are required.
2. Validated Packaging & Pre-Conditioning
Medical products are packed using:
- Insulated containers
- Gel packs or phase-change materials
- Tamper-evident seals
Packaging is pre-conditioned to the target temperature before loading to avoid thermal shock.
3. Refrigerated Transport & Handling
Products are transported using:
- Temperature-controlled vans or trucks
- Segregated compartments
- Clean, pharmaceutical-grade interiors
Drivers and handlers are trained in Good Distribution Practice (GDP) to minimize exposure during loading and unloading.
4. Real-Time Temperature Monitoring
Continuous monitoring is the backbone of medical cold chain transport. This includes:
- Digital data loggers
- IoT sensors with GPS tracking
- Automated alerts for temperature deviations
These systems provide end-to-end traceability, which is essential for audits and compliance.
5. Documentation, Verification & Delivery
Upon delivery:
- Temperature logs are reviewed
- Chain-of-custody is verified
- Proof of compliance is documented
If any deviation occurs, predefined corrective actions are triggered.
Why Medical Cold Chain Transport Is Critical for Healthcare
Medical cold chain failures can result in:
- Reduced drug efficacy
- Patient safety risks
- Regulatory penalties
- Financial losses
For example, a single vaccine shipment compromised by temperature deviation can cost thousands of dollars and delay immunization programs. This is why healthcare providers increasingly rely on specialized logistics partners like HEW Transportation, whose expertise lies in temperature-controlled transport, real-time monitoring, and regulatory compliance.
Industries That Depend on Medical Cold Chain Logistics
Medical cold chain transport supports multiple healthcare-related sectors, including:
- Pharmaceutical manufacturing
- Hospitals and clinics
- Diagnostic laboratories
- Biotechnology companies
- Medical research institutions
For a broader overview, see our in-depth guide on industries that need cold chain logistics.
Active vs Passive Medical Cold Chain Transport
Active Cold Chain
- Refrigerated vehicles
- Continuous power supply
- Best for long distances and high-value goods
Passive Cold Chain
- Insulated packaging with coolants
- Limited duration
- Ideal for short routes or last-mile delivery
| Feature | Active | Passive |
|---|---|---|
| Temperature Control | Continuous | Time-limited |
| Monitoring | Real-time | Logger-based |
| Cost | Higher | Lower |
| Risk Level | Minimal | Moderate |
Common Challenges in Medical Cold Chain Transport
Temperature Excursions
Caused by:
- Poor handling
- Equipment failure
- Traffic delays
Regulatory Compliance
Medical transport must meet:
- GDP standards
- Local health authority regulations
- Audit documentation requirements
Last-Mile Delivery Risks
The final delivery stage is often the highest risk point due to exposure during unloading, handover delays, and environmental temperature fluctuations. During last-mile delivery, temperature-sensitive medical products are most vulnerable because they are temporarily removed from controlled environments, increasing the likelihood of temperature excursions.
Best Practices for Reliable Medical Cold Chain Transport
- Choose GDP-compliant logistics providers
- Use validated packaging solutions
- Implement real-time monitoring
- Train staff in pharmaceutical handling
- Maintain complete documentation
Conclusion: Choosing the Right Medical Cold Chain Partner
Medical cold chain transport is not optional, it is a critical safeguard for patient safety and regulatory compliance. From planning and packaging to monitoring and final delivery, every step must be precise, validated, and fully traceable to maintain the integrity of temperature-sensitive medical products. At HEW Transportation, we specialize in medical and pharmaceutical cold chain logistics, combining temperature-controlled fleets, real-time monitoring systems, and industry-compliant operational processes to ensure medical products are transported safely, consistently, and in accordance with regulatory standards. For organizations managing temperature-sensitive medical deliveries, partnering with an experienced cold chain logistics provider ensures long-term reliability, compliance, and confidence across the entire healthcare supply chain.
FAQs (Frequently Asked Questions)
What happens if medical products exceed the required temperature range?
If temperature excursions occur, products may be quarantined, tested, or discarded depending on stability data and regulatory guidelines.
How is temperature monitored during transport?
Through digital data loggers, GPS-enabled sensors, and automated alert systems that track temperature in real time.
Is medical cold chain transport only for vaccines?
No. It also applies to biologics, insulin, blood products, diagnostic kits, and certain medical devices.
How long can products stay cold in passive packaging?
Typically 24–72 hours, depending on insulation quality and external conditions.
Why should businesses use specialized cold chain logistics providers?
Specialists ensure compliance, reduce risk, and protect product integrity—critical in healthcare logistics.






